|
|
21 CFR 820.100(a)(1) Each manufacturer shall establish and maintain procedures for implementing corrective and preventive action. The procedures shall include requirements for:
(1) Analyzing processes, work operations, concessions, quality audit reports, quality records, service records, complaints, returned product, and other sources of quality data to identify existing and potential causes of nonconforming product, or other quality problems. Appropriate statistical methodology shall be employed where necessary to detect recurring quality problems.
|
CAPA Analyze Phase Medical device, pharmaceutical, and biotechnology product and process issues are identified in many ways, including:
Inadequate responses to managing these issues, in particular customer complaints, and not performing robust risk analysis against them will result in a significant regulatory citing. Equally important is the ability to provide clear, objective evidence that your organization has handled and corrected the issue sufficiently.
It is important to have a central repository for the multiple sources of product, process and quality issues. A central location allows you to have a single source of the truth; to transparently supply evidence, on demand, that all issues / signals were identified, triaged, and analyzed for risk by the appropriate colleagues. This allows you to manage the workflow around issue analysis, as well as tracking the active decision and rationale of whether or not to generate a CAPA.
Well-managed quality incidents do not always result in a CAPA, but if one is required, creation of the CAPA is the next step in the process flow management.
The ExtraView Difference
ExtraView Enterprise serves as the information consolidator and becomes the central repository for multiple sources of product, process and quality issues. The ExtraView platform allows you to have a single source of the truth, to transparently supply evidence on demand that all issues / problems were identified, triaged, and analyzed for risk by the appropriate stakeholders. It also manages the workflow around the analysis, as well as the decision and rationale of whether or not to generate a CAPA. ExtraView's built-in reporting and notification mechanisms allow the analysis to be communicated to all parties.
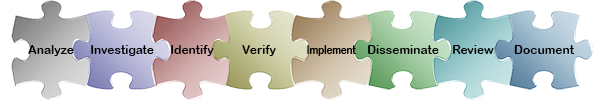
Please select the Investigate link to move to the next phase of the CAPA process, or select any of the puzzle pieces below to learn more about how ExtraView meets your business requirements in that specific CAPA area. For a synopsis of ExtraView’s role in the entire CAPA process, see the ExtraView CAPA Functionality Matrix.
Example Screens from Customer Implementations
Learn About Other CAPA Phases
|
|





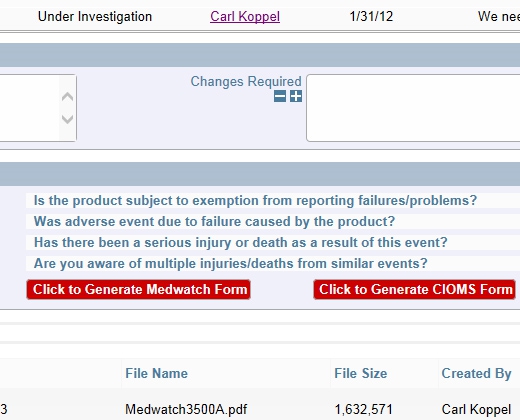 Example Decision Tree
Example Decision Tree Completely Configurable Screens
Completely Configurable Screens Gather Information from Multiple Sources
Gather Information from Multiple Sources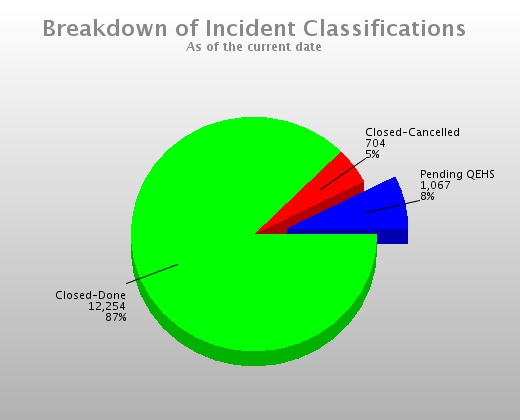 Full Reporting Capabilities Including Charts
Full Reporting Capabilities Including Charts CAPAs Follow Any Workflow You Require
CAPAs Follow Any Workflow You Require