|
|
21 CFR 820.100(a)(4) Each manufacturer shall establish and maintain procedures for implementing corrective and preventive action. The procedures shall include requirements for:
(4) Verifying or validating the corrective and preventive action to ensure that such action is effective and does not adversely affect the finished device (or product).
|
CAPA Verification & Validation Phase
Verification addresses whether a product or process meets its intended use or requirements. Validation assures that there is quality in the process or building of the product itself. In Life Sciences, you are required to provide evidence of compliance to verification and validation standards.
CAPAs require verification and validation that both the original issue was addressed and that no other quality issues were introduced in the process.
The ExtraView Difference
As part of a comprehensive CAPA plan within ExtraView Enterprise, you will outline the approach that will be taken to verify and validate each corrective action. You will track the dates when testing was completed and the overall results. If desired, ExtraView Enterprise can store documentation on test planning, test execution and outcomes as proof of effectiveness (e.g. IQs, OQs, and PQs for devices). The solution also easily references links to documentation in external source repositories.
Please select the Implementation link to move to the next CAPA phase, or select any of the puzzle pieces below to learn more about how ExtraView meets your business requirements in that specific CAPA area. For a synopsis of Extraview’s role in the entire CAPA process, see the ExtraView CAPA Functionality Matrix.
Example Screens from Customer Implementations
Learn About Other CAPA Phases
|
|





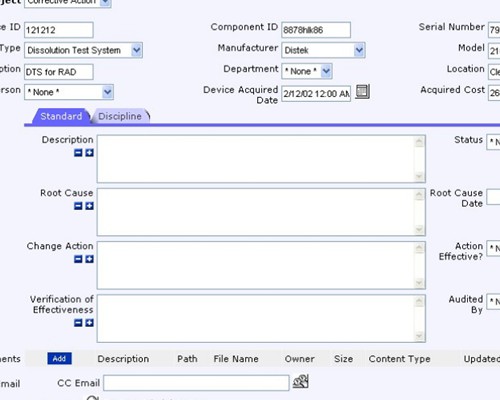 Use your process to provide complete traceability of results
Use your process to provide complete traceability of results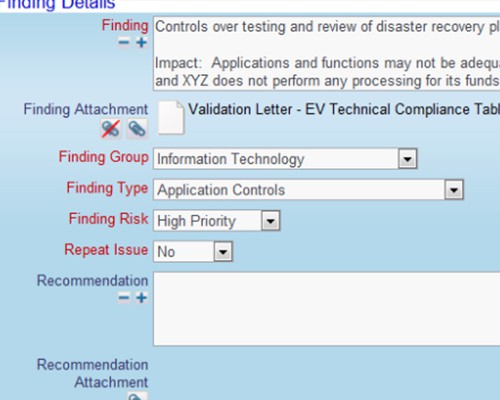 Record your findings as part of the verification process
Record your findings as part of the verification process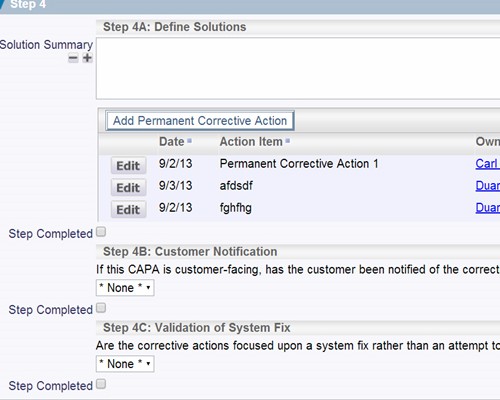 Record the details of the corrective actions that were implemented
Record the details of the corrective actions that were implemented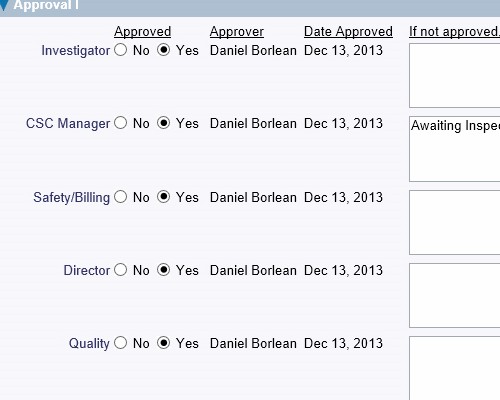 Record who verified the issue and when it was verified
Record who verified the issue and when it was verified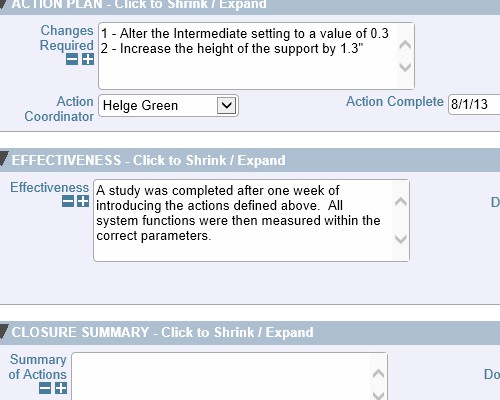 Thoroughly check how well the actions performed
Thoroughly check how well the actions performed